Assistant Professor of Developmental Biology
Research Interests
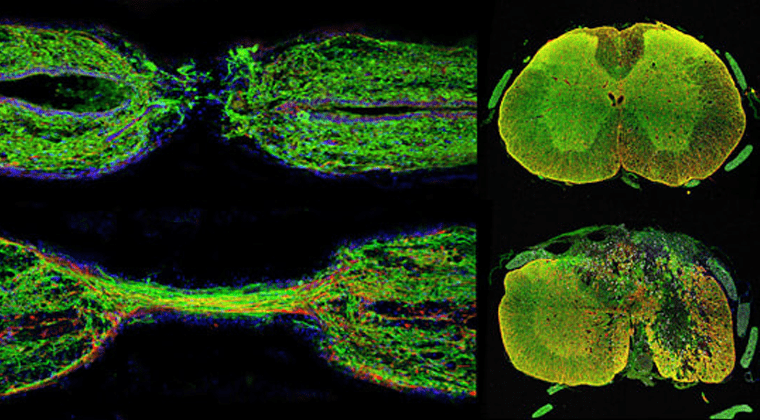
My lab aims to elucidate evolutionarily conserved mechanisms of spinal cord regeneration, and to develop zebrafish-inspired interventions to promote spinal cord repair in mammals. Adult zebrafish possess an elevated regenerative capacity and lack the anti-regenerative complications displayed after mammalian spinal cord injury. Our goal is to leverage the strengths of the zebrafish model system to uncover the molecular identities of pro-regenerative cells in zebrafish, and to reconstruct analogous identities in humans.
In zebrafish, specialized bridging glia connect the transected spinal cord and support axon regrowth across the lesion. We are pursuing a number of transcriptomic and genetic approaches to elucidate glial bridging mechanisms in zebrafish. We are using comparative transcriptomic to uncover the bases for differential regenerative capacity between the highly regenerative zebrafish and poorly regenerative mouse models, and glial cell reprogramming to engineer human cells into zebrafish-like bridging cells to promote spinal cord repair in mammals.
Adult zebrafish are also capable of adult neurogenesis in response to injury. Ongoing studies in the lab aim to identify new regulators of neurogenesis and functional repair, and to trace the contributions of ependymal progenitors to neurogenesis in the adult spinal cord.
Professional Education
- BSc: American University of Beirut
- PhD: Genetics and Development, 2005-2010
- MS: American University of Beirut, 2003-2005
- Postdoctoral Fellow: Duke University Medical Center, 2012-2017
- Postdoctoral Fellow: UT Southwestern Medical Center, 2012-2017
Affiliations
- Hope Center for Neurological Disorders
- Developmental Biology
Mokalled Lab
The Mokalled lab aims to elucidate evolutionarily conserved mechanisms of spinal cord regeneration, and to develop zebrafish-inspired interventions to promote spinal cord repair in mammals. Our goal is to leverage the strengths of the zebrafish model system to uncover the molecular identities of pro-regenerative cells in zebrafish, and to reconstruct analogous identities in humans.
